Author: Grzegorz Grzybowski
Tablet Compression Process Engineer | Emerson
Specialization: Pharma 4.0, Digitalization, Innovation
Experience gained at Natoli, Prodieco, Adamus HT
Co-creator of TechPointCloud – a digital tool for the inspection of tablet press tooling.

Introduction
The tablet—today the most common dosage form—has over 180 years of technological development behind it. Its origin is usually dated to 1843, when William Brockedon first compressed powder into a coherent unit dose. From that moment, both the form itself and the techniques for making it have been continuously improved. A breakthrough came with rotary presses and, alongside them, the evolution of tools: punches and dies that enabled controlled, high-throughput production.
In 2024, more than 5 trillion tablets were produced worldwide, most of them in India and China. In Poland—over 3.7 billion. This form’s popularity stems from its stability, ease of administration, low production cost, and precise dosing. Achieving this, however, requires exceptionally rigorous quality control—not only of APIs and excipients, but also of the tooling that directly contacts the product.
Punches and dies are fundamental elements of any tablet press. Their job is not only to form a medicinal product or dietary supplement, but also to ensure correct tablet mass, shape, surface smoothness, and—where relevant—coating integrity. Material fatigue, micro-damage, scratches, or deformation can lead to serious tablet defects, such as capping, picking, or sticking.
Accordingly, proper tool control is essential. The traditional approach centers on measurements—length, diameter, cup depth—taken manually or with automated systems. In practice, however, systematic organoleptic inspection—visual and tactile assessment of roughness, scratches, or unnatural surface reflections—often reveals abnormalities earliest.
Organoleptic inspection of punches and dies exposes not only tool wear; it is often the first signal of formulation issues: e.g., insufficient lubrication, excessive dusting, or granule non-uniformity. It can also reveal press faults—wear of the rotor, seals, cams—or assembly errors and improper washing processes.
Experience across many plants shows that relying solely on numerical measurement data can be inadequate. A tool that “meets tolerance” may still generate undesirable effects during compression. Therefore, more organizations are adopting a structured approach to visual inspection, treating it as the primary element of their tooling management system.
This article explains why well-executed organoleptic inspection—supported by process data, formulation knowledge, and a tooling management system—becomes a practical optimization lever, extending punch and die life, reducing downtime and rejects, and improving overall tableting efficiency.
The Role of Tool Inspection in GMP Practice
In GMP-compliant (and any) production, the condition of punches and dies directly affects tablet quality. Even minor damage can cause product defects and batch losses.
Organoleptic inspection—visual and tactile—allows rapid detection of wear, damage, or surface irregularities. It often proves more effective than geometric measurements, which are sometimes performed merely as a formality and rarely analyzed further. For example, a punch length check may not reveal engraving damage or micro-cracks on edges, which are visible at a glance. Organoleptic assessment, by contrast, yields actionable insights and enables faster, more accurate responses.
Embedding organoleptic inspection into standard quality procedures is the foundation of a modern system for managing compression tooling. Visual checks and touch enable operators to rapidly catch even subtle changes in punch or die surfaces—e.g., fine scratches, land irregularities, micro-cracks. Unlike geometric measurements, which may be infrequent and treated as tick-box exercises, organoleptic inspection directly guides interventions. Documenting inspection outcomes within auditable procedures boosts GMP compliance and supports decisions on repair, replacement, or rotation. Such inspection should be treated as the first stage of tooling life-cycle management, introducing decision points and trend tracking of wear. The effect: better preventive planning, reduced downtime, and significant material savings in the tableting process.
From the Shop Floor — Common Damage Types and Their Causes
Damage to punches and dies is a well-known phenomenon in tablet manufacturing, with direct impact on product quality and process stability. It most often results from assembly errors, improper storage, cursory maintenance, or lack of thorough post-campaign inspection. Cleaning is a particularly vulnerable stage. Even minor tool damage can trigger tablet defects, process disturbances, or unplanned stoppages. Regular technical checks, grounded in operator experience, should be a permanent element of tablet press tooling oversight. Shifting effort from formal, low-utility measurements toward systematic organoleptic inspection translates into tangible business benefits—both quality and operational.
Below are example images of punch and die damage with brief descriptions. These are illustrative cases only—each pharmaceutical company should document its own experiences, ideally in an organized repository of photos and technical notes, and treat them as the foundation for building internal tooling management know-how.

Figure 1. Damage to the working edge of a punch
The image shows damage to the working edge of a punch, most likely caused by improper mounting or removal of the tool in the press rotor. The damage may result from not using an alignment fixture or from using non-recommended auxiliary tools. Also consider transport, cleaning, or storage as potential causes—especially impact on hard surfaces, contact with metal components, or improper placement in a tool cassette.

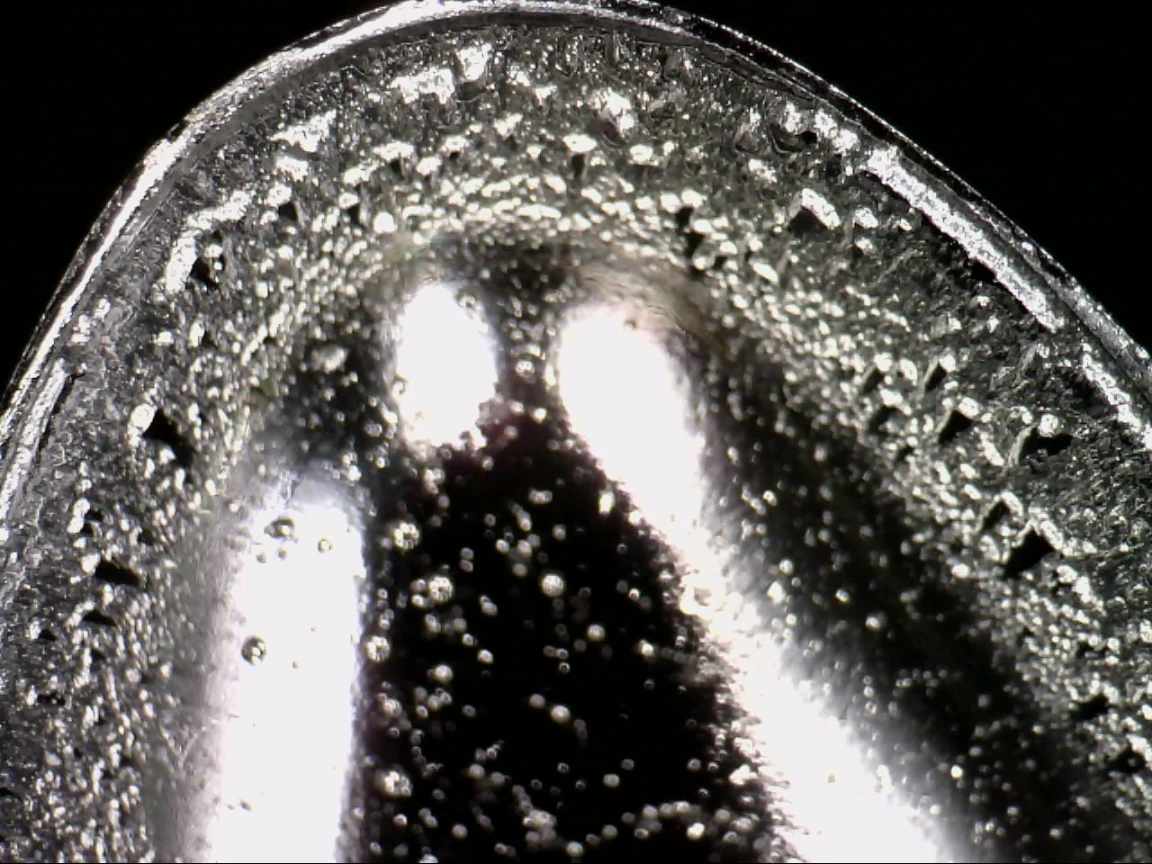
Figure 2. Galling of the lower punch in the die due to a low-melting API
The image shows typical galling marks inside the die. The damage results from softening of a low-melting active ingredient, leading to adhesion of material to the punch flank and the die’s internal working surface. Characteristic friction marks and localized product deposits indicate hindered material release during compression. This can increase compression forces, destabilize tablet weight, and accelerate tool wear.

Figure 3. Punch head damage caused by compression system operating errors
Visible symptoms stem from inadequate preparation of the compression roll assembly and lubrication intervals that do not match load and machine speed. Damage to the punch head surface includes wear, localized scratches, and signs of thermal distress. Such defects can cause uneven force distribution and accelerated cam wear. Aligning lubrication intervals with real process conditions is crucial to prevent similar failures.

Figure 4. Geometric damage to a punch caused by mounting/removal errors
The image shows tip damage typically arising from careless mounting or removal of the tool in the press rotor. Such damage can also result from non-compliance with tooling maintenance procedures, e.g., improper storage or use of unsuitable auxiliary tools. In most cases, this defect disqualifies the tool from further use.

Figure 5. Surface wear of the punch cup typical for formulations with high solid content
The image shows surface degradation of the working layer caused by prolonged contact with a highly abrasive granulate. This type of damage is typical for formulations containing many solids with irregular, sharp edges that mechanically abrade the metal surface. Visible matting, localized losses, and micro-scratches degrade tool performance—increasing friction, hindering mass release, and raising the risk of product sticking. The degradation can also promote deposit buildup and disrupt compression, especially at high rotor speeds and elevated granule humidity. Regular visual assessment of the working surface and appropriate selection of protective coatings or base materials are key to limiting this wear.

Figure 6. Ring-shaped wear mark on the die’s internal surface
The visible ring formed due to long-term contact of the die’s internal working surface with a highly abrasive granulate. The characteristic pattern results from intense, cyclic friction of sharp-edged solids at high press speeds. In the affected zone one observes loss of smoothness, fine scratches, and local matting, which can disturb uniform mass flow and increase frictional forces.

Figure 7. Punch cup wear due to granulate with an aggressive physical structure
A typical case of progressive working-surface wear caused by repetitive contact with granulates containing hard, aggressive components (e.g., mineral salts, inorganic pigments, some plant extracts). Symptoms include micro-scratches, local matting, initial loss of smoothness, and irregular friction marks in the contact zone. Such wear raises the risk of product adhesion (sticking) and lowers tablet aesthetics.
Based on these damage cases, several technical recommendations and preventive actions significantly increase tooling durability and process stability. Regular organoleptic assessment of working surfaces is crucial—the simplest and most effective method for early detection of defects such as deformation, micro-scratches, adhesion, or heat marks—before they affect tablet parameters. Proper press set-up is equally important, including checks of rotor and compression roll condition, and use of appropriate mounting tools, especially for shaped punches. Material selection for punches and dies should suit granulate properties—formulations with hard, abrasive components call for higher-wear-resistance materials or protective coatings that minimize friction and extend tool life. These measures should be complemented by optimized lubrication plans for the compression system. Equally critical are precise and consistently followed washing and drying procedures—errors at this stage (residual moisture, aggressive detergents, improper drying) can cause corrosion and surface oxidation. At every stage of working with punches and dies, mechanical damage can occur.
In the longer term, an electronic system for tooling oversight and record-keeping becomes indispensable in modern management. Such solutions enable full life-cycle control, recording inspection history, tracking wear, planning refurbishment, and analyzing root causes of recurring failures. Implementing a tooling management system today is not just a quality aid—it is a necessary step for any organization striving for stability, predictability, and GMP-compliant production. An integrated approach—rooted in inspection, prevention, data, and system oversight—forms the foundation of effective tableting process management.
The Digital Transformation of Tooling Management — From Visual Checks to Predictive Systems
Tooling management in the pharmaceutical industry is entering a phase of deep digital transformation. Solutions based on data integration, prediction, and automation are gaining prominence. Systems leveraging artificial intelligence (AI), historical data analytics, and sensors integrated with tablet presses play a key role. This enables not only real-time monitoring of tool wear but also forecasting replacement timing—aligned with predictive maintenance concepts long established in other industries.
Modern systems connect information from MES, ERP, and LIMS to automatically detect deviations, generate alerts, and support service decisions. Intelligent analytics optimize maintenance planning, reduce costs, and minimize the risk of production downtime.
In parallel, tools for standardizing and transferring operational knowledge are increasingly important. Solutions that document observations and case analyses support competency growth within the organization. In this context, organoleptic inspection does not lose relevance—quite the opposite. It remains the practical first-line method, increasingly supported by technology and expertise, making it an integral element of modern tooling control.
TechPoint — Easy Tableting Process
In response to the challenges of modern tablet production, our team developed a free cloud tool that streamlines punch and die assessment and enables ongoing documentation. It supports daily practice with digital organoleptic inspection, damage recording, and efficient information management. TechPoint is a highly practical aid for tableting—designed with end users and academic environments in mind.
TechPoint — Easy Tableting Process also proposes a management philosophy built on observation, reducing low-value measurements, and strengthening visual control. This approach not only supports GMP compliance but also measurably improves operational efficiency and finished product quality.
More information can be found at techpointcloud.pl. We invite you to register and use the free software, backed by an experienced team of practitioners and scientists. Our solution offers: ease of use, time savings, expert support, and access to proven know-how.
Conclusion
Modern tooling management in tableting requires more than adherence to GMP norms and procedures; it demands a flexible approach grounded in practical experience, technology, and analytics. Once marginalized, organoleptic inspection has become the cornerstone of effective tooling control and a source of valuable know-how. Augmenting it with digital tools and predictive models enables not only better responses to tool wear but, crucially, proactive action.
Industry pressures—rising quality expectations, shorter campaign times, and the need to minimize losses—require a new mindset. It is time to make tooling inspection a real element of strategic production management, not just a checkbox on a to-do list.
To build the future of tableting, we should rely both on proven organoleptic methods and on innovative tools that make the entire process more predictable, safer, and optimized.